Molecular Mechanisms Underlying Mimosa acutistipula Success in Amazonian Rehabilitating Minelands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Sampling and Analyses
2.2. Root Sampling
2.3. Protein Isolation
2.4. Protein Identification and Data Analysis
3. Results
3.1. Substrate Properties
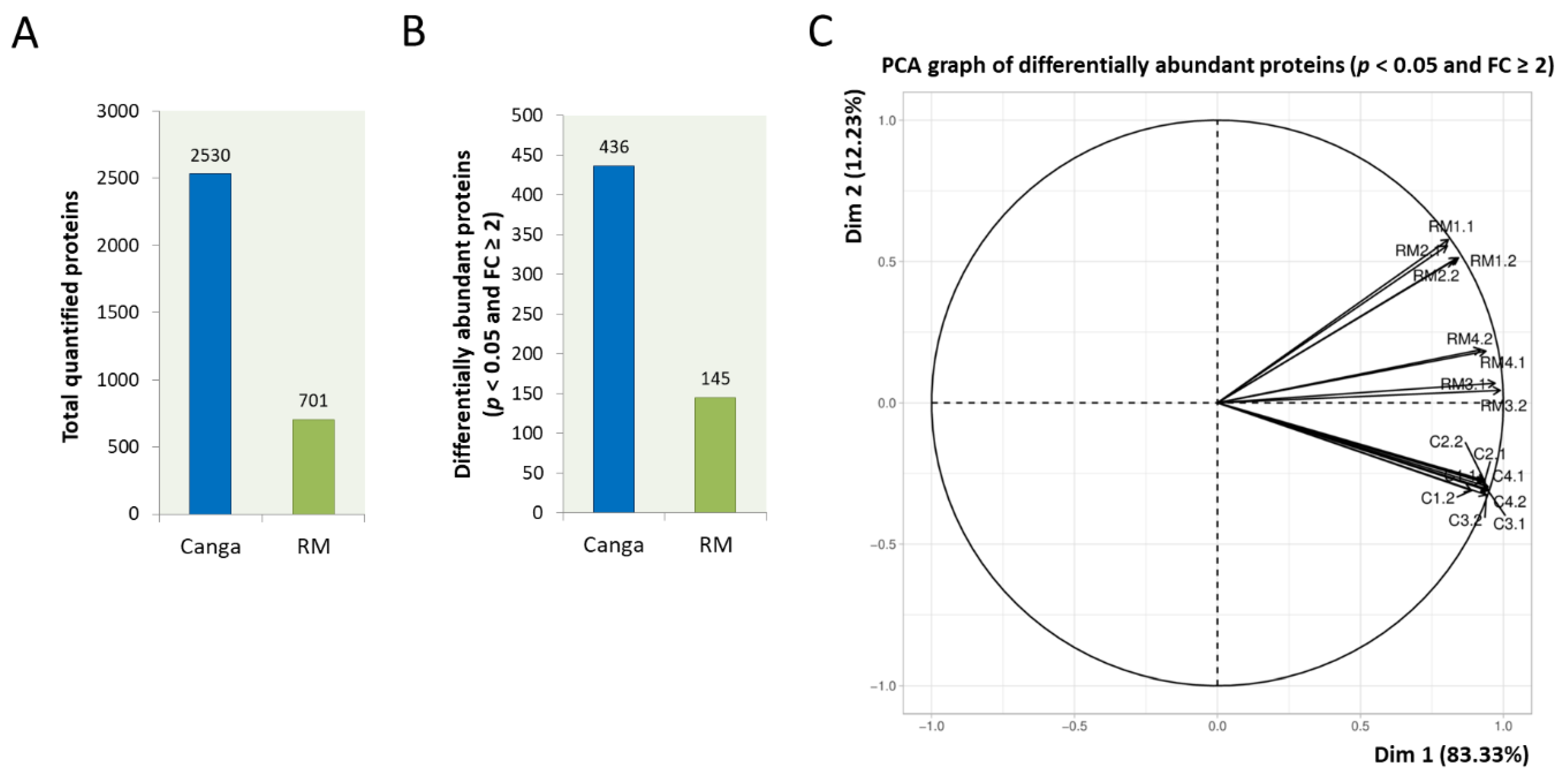
3.2. Protein Profiles
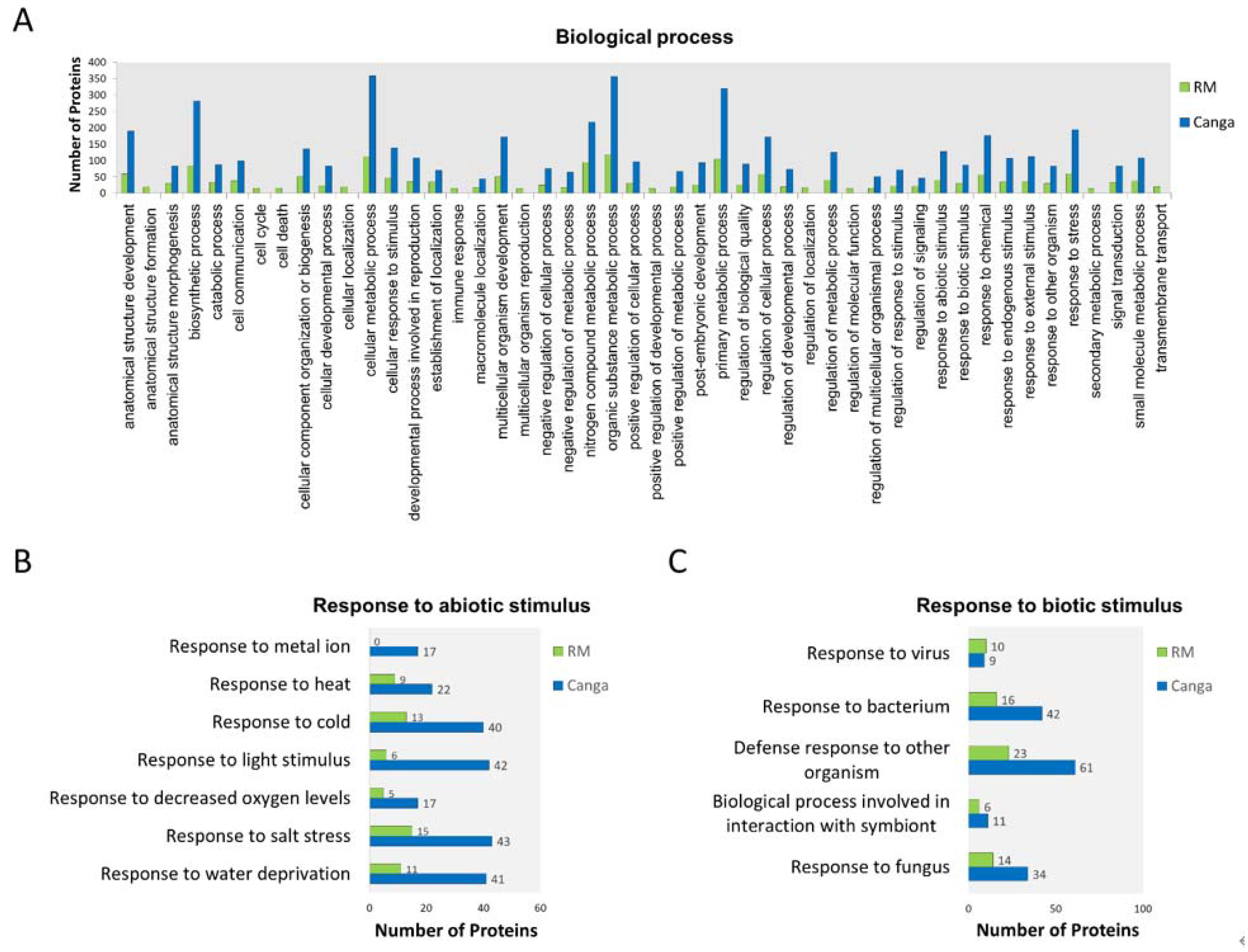
3.3. Gene Ontology Annotation
4. Discussion
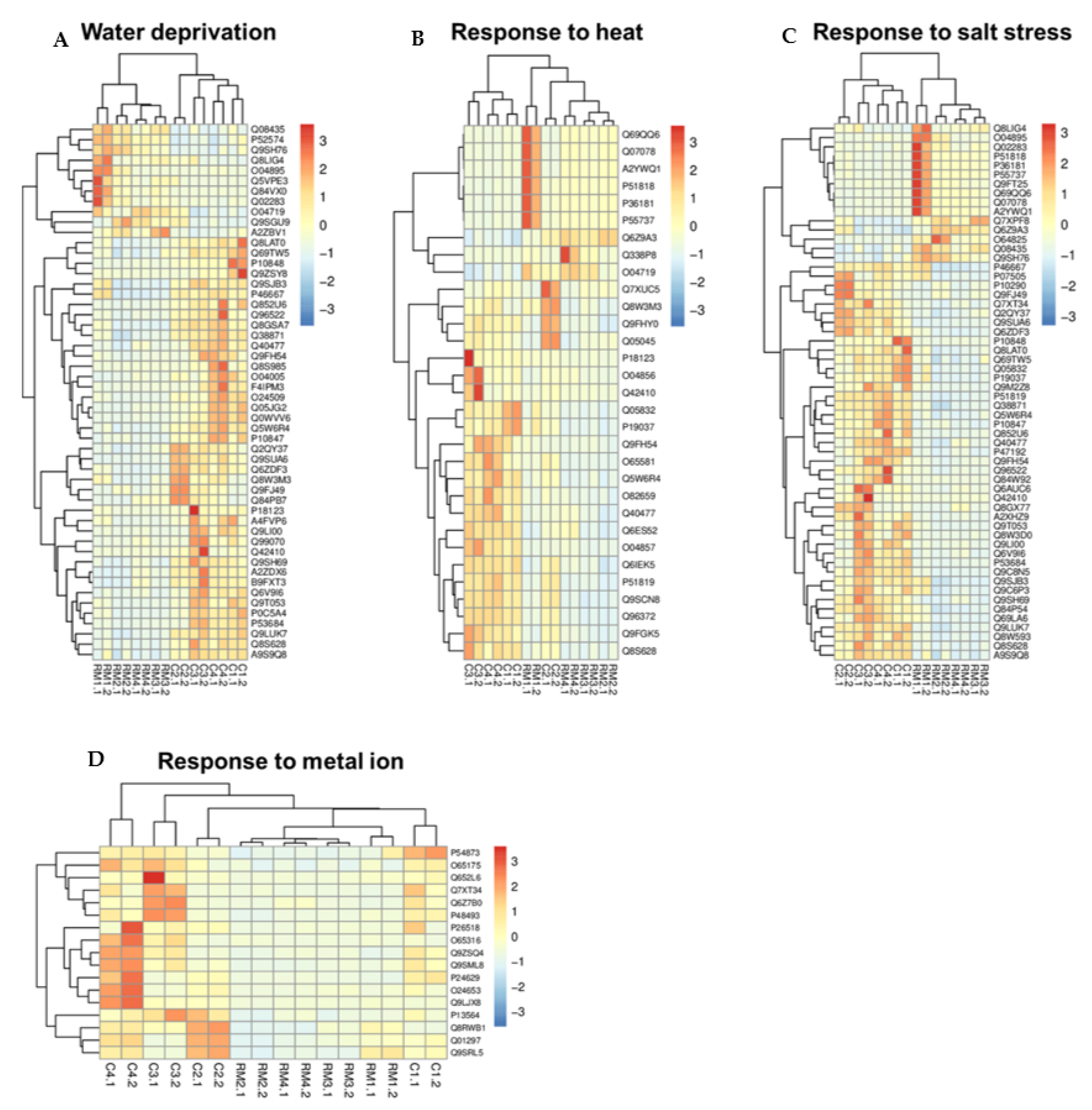
4.1. Proteins Related to Abiotic Stimulus
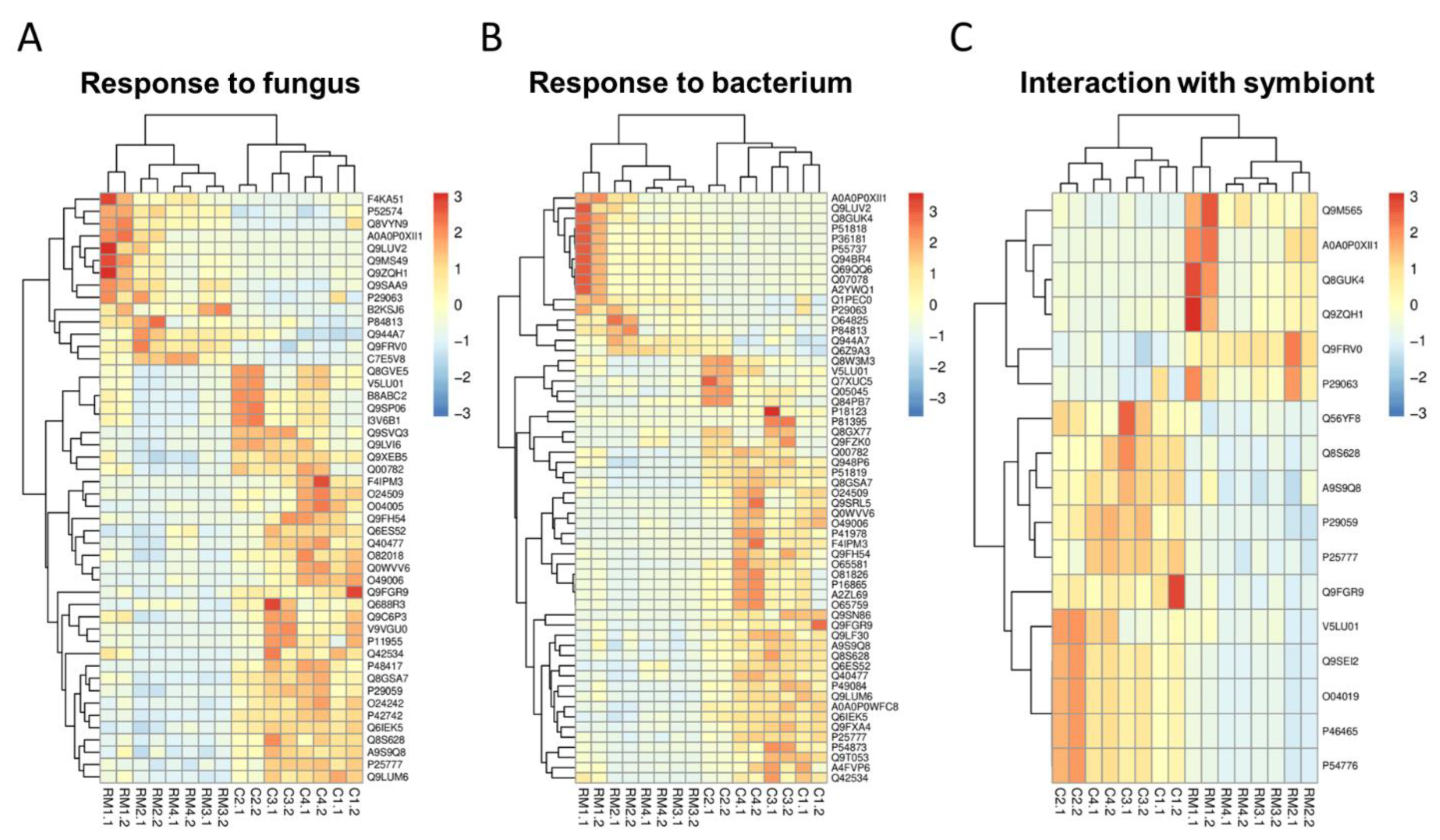
4.2. Proteins Related to Biotic Stimulus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Santos, R.S.P.; Milanez, B. The Global Production Network for iron ore: Materiality, corporate strategies, and social contestation in Brazil. Extr. Ind. Soc. 2015, 2, 756–765. [Google Scholar] [CrossRef]
- Jacobi, C.M.; Do Carmo, F.F.; Vincent, R.C.; Stehmann, J.R. Plant communities on ironstone outcrops: A diverse and endangered Brazilian ecosystem. Biodivers. Conserv. 2007, 16, 2185–2200. [Google Scholar] [CrossRef]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga biodiversity, a matter of mining. Front. Plant Sci. 2014, 5, 653. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.A.R.; do Carmo, F.F. ‘Quadrilátero Ferrífero’: A Beautiful and Neglected Landscape between the Gold and Iron Ore Reservoirs. In Landscapes and Landforms of Brazil, 1st ed.; Carvalho, B., Rodrigues, A., Cordeiro, L., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 319–330. [Google Scholar]
- Schaefer, C.E.; Cândido, H.G.; Corrêa, G.R.; Pereira, A.; Nunes, J.A.; Souza, O.F.; Marins, A.; Fernandes-Filho, E.; Ker, J.C. Solos desenvolvidos sobre canga ferruginosa no Brasil: Uma revisão crítica e papel ecológico de termiteiros. In Geossistemas Ferruginosos do Brasil: àreas Prioritárias Para Conservação da Diversidade Geológica e Biológica. Patrimônio Cultural e Serviço Ambiental; Carmo, F.F., Kamino, L.H.Y., Eds.; 3i Editora: Belo Horizonte, Brazil, 2015; pp. 77–102. [Google Scholar]
- Gastauer, M.; Silva, J.R.; Junior, C.F.C.; Ramos, S.J.; Souza Filho, P.W.M.; Neto, A.E.F.; Siqueira, J.O. Mine land rehabilitation: Modern ecological approaches for more sustainable mining. J. Clean. Prod. 2018, 172, 1409–1422. [Google Scholar]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar]
- Gastauer, M.; Souza Filho, P.W.M.; Ramos, S.J.; Caldeira, C.F.; Silva, J.R.; Siqueira, J.O.; Neto, A.E.F. Mine land rehabilitation in Brazil: Goals and techniques in the context of legal requirements. Ambio 2019, 48, 74–88. [Google Scholar] [CrossRef]
- Ribeiro, P.G.; Martins, G.C.; Gastauer, M.; da Silva Junior, E.C.; Santos, D.C.; Frois Caldeira Júnior, C.; Cavalcante, R.B.L.; dos Santos, D.S.; Carneiro, M.A.C.; Valadares, R.B.d.S. Spectral and soil quality index for monitoring environmental rehabilitation and soil carbon stock in an Amazonian sandstone mine. Sustainability 2022, 14, 597. [Google Scholar] [CrossRef]
- Zappi, D.; Gastauer, M.; Ramos, S.; Nunes, S.; Caldeira, C.; Souza-Filho, P.; Guimarães, J.; Giannini, T.; Viana, V.; Lovo, J. Plantas Nativas Para Recuperação de Áreas de Mineração em Carajás; Instituto Tecnológico Vale: Belém, Brazil, 2018; p. 282. [Google Scholar]
- Guedes, R.S.; Ramos, S.J.; Gastauer, M.; Júnior, C.F.C.; Martins, G.C.; da Rocha Nascimento Júnior, W.; de Souza-Filho, P.W.M.; Siqueira, J.O. Challenges and potential approaches for soil recovery in iron open pit mines and waste piles. Environmental Earth Sciences 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Marques, M.; Aguiar, C.R.C.; Silva, J.J.L.S.d. Desafios técnicos e barreiras sociais, econômicas e regulatórias na fitorremediação de solos contaminados. Rev. Bras. Ciência Solo 2011, 35, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gastauer, M.; Ramos, S.J.; Caldeira, C.F.; Siqueira, J.O. Reintroduction of native plants indicates the return of ecosystem services after iron mining at the Urucum Massif. Ecosphere 2021, 12, e03762. [Google Scholar] [CrossRef]
- Ramos, S.J.; Caldeira, C.F.; Gastauer, M.; Costa, D.L.P.; Neto, A.E.F.; de Souza, F.B.M.; Souza-Filho, P.W.M.; Siqueira, J.O. Native leguminous plants for mineland revegetation in the eastern Amazon: Seed characteristics and germination. New For. 2019, 50, 859–872. [Google Scholar] [CrossRef]
- Ramos, S.J.; Gastauer, M.; Mitre, S.K.; Caldeira, C.F.; Silva, J.R.; Neto, A.E.F.; Oliveira, G.; Souza Filho, P.W.; Siqueira, J.O. Plant growth and nutrient use efficiency of two native Fabaceae species for mineland revegetation in the eastern Amazon. J. For. Res. 2019, 31, 2287–2293. [Google Scholar]
- Silva, J.R.; Gastauer, M.; Ramos, S.J.; Mitre, S.K.; Neto, A.E.F.; Siqueira, J.O.; Caldeira, C.F. Initial growth of Fabaceae species: Combined effects of topsoil and fertilizer application for mineland revegetation. Flora 2018, 246, 109–117. [Google Scholar]
- Carvalho, C.S.; Forester, B.R.; Mitre, S.K.; Alves, R.; Imperatriz-Fonseca, V.L.; Ramos, S.J.; Resende-Moreira, L.C.; Siqueira, J.O.; Trevelin, L.C.; Caldeira, C.F. Combining genotype, phenotype, and environmental data to delineate site-adjusted provenance strategies for ecological restoration. Mol. Ecol. Resour. 2021, 21, 44–58. [Google Scholar] [PubMed]
- Nunes, J.A.; Schaefer, C.E.; Ferreira Júnior, W.G.; Neri, A.V.; Correa, G.R.; Enright, N.J. Soil-vegetation relationships on a banded ironstone ‘island’, Carajás Plateau, Brazilian Eastern Amazonia. An. Acad. Bras. Ciências 2015, 87, 2097–2110. [Google Scholar]
- Giannini, T.C.; Giulietti, A.M.; Harley, R.M.; Viana, P.L.; Jaffe, R.; Alves, R.; Pinto, C.E.; Mota, N.F.; Caldeira, C.F., Jr.; Imperatriz-Fonseca, V.L. Selecting plant species for practical restoration of degraded lands using a multiple-trait approach. Austral Ecol. 2017, 42, 510–521. [Google Scholar]
- Nascimento, S.V.; Oliveira Costa, P.H.; Herrera, H.; Caldeira, C.F.; Gastauer, M.; Ramos, S.J.; Oliveira, G.; Valadares, R. Proteomic Profiling and Rhizosphere-Associated Microbial Communities Reveal Adaptive Mechanisms of Dioclea Apurensis Kunth in Eastern Amazon’s Rehabilitating Minelands. Plants 2022, 11, 712. [Google Scholar] [CrossRef]
- Costa, P.H.d.O.; Nascimento, S.V.d.; Herrera, H.; Gastauer, M.; Ramos, S.J.; Caldeira, C.F.; Oliveira, G.; Valadares, R.B.d.S. Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland. Processes 2021, 9, 2079. [Google Scholar]
- Kalubi, K.; Michael, P.; Omri, A. Analysis of gene expression in red maple (Acer rubrum) and trembling aspen (Populus tremuloides) populations from a mining region. Genes Genom. 2018, 40, 1127–1136. [Google Scholar]
- Husain, R.; Weeden, H.; Bogush, D.; Deguchi, M.; Soliman, M.; Potlakayala, S.; Katam, R.; Goldman, S.; Rudrabhatla, S. Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS ONE 2019, 14, e0221570. [Google Scholar]
- Berka, M.; Luklová, M.; Dufková, H.; Berková, V.; Novák, J.; Saiz-Fernández, I.; Rashotte, A.M.; Brzobohatý, B.; Černý, M. Barley root proteome and metabolome in response to cytokinin and abiotic stimuli. Front. Plant Sci. 2020, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Di Zinno, J.; Montanini, B.; Manfredi, M.; Marengo, E.; Fornasier, F.; Ferrarini, A.; Careri, M.; Visioli, G. Biostimulants applied to maize seeds modulate the enzymatic activity and metaproteome of the rhizosphere. Appl. Soil Ecol. 2020, 148, 103480. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Feng, Y.; Sikandar, A.; Zhu, X.; Fan, H.; Liu, X.; Chen, L.; Duan, Y. iTRAQ-Based Proteomic Analysis Reveals the Role of the Biological Control Agent, Sinorhizobium fredii Strain Sneb183, in Enhancing Soybean Resistance against the Soybean Cyst Nematode. Front. Plant Sci. 2020, 2002, 597819. [Google Scholar] [CrossRef] [PubMed]
- Trindade, F.C.; Ramos, S.J.; Gastauer, M.; Saraiva, A.M.M.; Caldeira, C.F.; Oliveira, G.; da Silva Valadares, R.B. Metaproteomes reveal increased capacity for stress tolerance of soil microbes in ferruginous tropical rocky outcrops. Pedobiologia 2020, 81, 150664. [Google Scholar] [CrossRef]
- Kettler, T.; Doran, J.W.; Gilbert, T. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef] [Green Version]
- Gastauer, M.; de Medeiros Sarmento, P.S.; Santos, V.C.A.; Caldeira, C.F.; Ramos, S.J.; Teodoro, G.S.; Siqueira, J.O. Vegetative functional traits guide plant species selection for initial mineland rehabilitation. Ecol. Eng. 2020, 148, 105763. [Google Scholar] [CrossRef]
- Viana, P.L.; Mota, N.F.d.O.; Gil, A.d.S.B.; Salino, A.; Zappi, D.C.; Harley, R.M.; Ilkiu-Borges, A.L.; Secco, R.d.S.; Almeida, T.E.; Watanabe, M.T.C. Flora das cangas da Serra dos Carajás, Pará, Brasil: História, área de estudos e metodologia. Rodriguésia 2016, 67, 1107–1124. [Google Scholar]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 2006, 27, 2782–2786. [Google Scholar] [CrossRef]
- Nascimento, S.V.; Magalhaes, M.M.; Cunha, R.L.; de Oliveira Costa, P.H.; de Oliveira Alves, R.C.; de Oliveira, G.C.; da Silva Valadares, R.B. Differential accumulation of proteins in oil palms affected by fatal yellowing disease. PLoS ONE 2018, 13, e0195538. [Google Scholar] [CrossRef] [Green Version]
- Herrera, H.; Valadares, R.; Oliveira, G.; Fuentes, A.; Almonacid, L.; do Nascimento, S.V.; Bashan, Y.; Arriagada, C. Adaptation and tolerance mechanisms developed by mycorrhizal Bipinnula fimbriata plantlets (Orchidaceae) in a heavy metal-polluted ecosystem. Mycorrhiza 2018, 28, 651–663. [Google Scholar] [CrossRef]
- Varotto, S.; Tani, E.; Abraham, E.; Krugman, T.; Kapazoglou, A.; Melzer, R.; Radanović, A.; Miladinović, D. Epigenetics: Possible applications in climate-smart crop breeding. J. Exp. Bot. 2020, 71, 5223–5236. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 1–14. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Kobayashi, Y.; Iuchi, S.; Koyama, H. Synergistic and antagonistic pleiotropy of STOP1 in stress tolerance. Trends Plant Sci. 2021, 26, 1014–1022. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Belachew, A.; Ma, S.F.; Young, M.; Ade, J.; Shen, Y.; Marion, C.M.; Holtan, H.E.; Bailey, A.; Stone, J.K. The EDLL motif: A potent plant transcriptional activation domain from AP2/ERF transcription factors. Plant J. 2012, 70, 855–865. [Google Scholar] [CrossRef]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Alemán, F. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 2015, 4, e03599. [Google Scholar] [CrossRef]
- Atif, R.M.; Shahid, L.; Waqas, M.; Ali, B.; Rashid, M.A.R.; Azeem, F.; Nawaz, M.A.; Wani, S.H.; Chung, G. Insights on calcium-dependent protein kinases (CPKs) signaling for abiotic stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 5298. [Google Scholar] [CrossRef] [Green Version]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Zhao, Y.; Tang, L.; Zhang, R.; Sun, M.; Guo, H.; Kong, X.; Li, A.; Mao, L. Molecular evolution of two duplicated CDPK genes CPK7 and CPK12 in grass species: A case study in wheat (Triticum aestivum L.). Gene 2011, 475, 94–103. [Google Scholar] [CrossRef]
- Li, G.; Boudsocq, M.; Hem, S.; Vialaret, J.; Rossignol, M.; Maurel, C.; Santoni, V. The calcium-dependent protein kinase CPK 7 acts on root hydraulic conductivity. Plant Cell Environ. 2015, 38, 1312–1320. [Google Scholar] [CrossRef]
- Kim, K.-N. Stress responses mediated by the CBL calcium sensors in plants. Plant Biotechnol. Rep. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Tang, R.-J.; Wang, C.; Li, K.; Luan, S. The CBL–CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, F.A.; Estrada, Y.; Flores, F.B.; Ortíz-Atienza, A.; Lozano, R.; Egea, I. The Ca2+ Sensor Calcineurin B–Like Protein 10 in Plants: Emerging New Crucial Roles for Plant Abiotic Stress Tolerance. Front. Plant Sci. 2021, 2155, 599944. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.; Goodger, J.Q.; Alvarez, S.; Marsh, E.L.; Berla, B.; Lockhart, E.; Jung, J.; Li, P.; Bohnert, H.J.; Schachtman, D.P. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 2010, 61, 3395–3405. [Google Scholar] [CrossRef] [Green Version]
- Jarzyniak, K.M.; Jasiński, M. Membrane transporters and drought resistance–a complex issue. Front. Plant Sci. 2014, 5, 687. [Google Scholar] [CrossRef] [Green Version]
- Daszkowska-Golec, A. The role of abscisic acid in drought stress: How aba helps plants to cope with drought stress. In Drought Stress Tolerance in Plants, Vol 2; Springer: New York, NY, USA, 2016; pp. 123–151. [Google Scholar]
- Hou, Y.-J.; Zhu, Y.; Wang, P.; Zhao, Y.; Xie, S.; Batelli, G.; Wang, B.; Duan, C.-G.; Wang, X.; Xing, L. Type one protein phosphatase 1 and its regulatory protein inhibitor 2 negatively regulate ABA signaling. PLoS Genet. 2016, 12, e1005835. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Nguyen, N.H.; Cheong, J.-J. Transcriptional Regulation of Protein Phosphatase 2C Genes to Modulate Abscisic Acid Signaling. Int. J. Mol. Sci. 2020, 21, 9517. [Google Scholar] [CrossRef]
- Goodger, J.Q.; Schachtman, D.P. Re-examining the role of ABA as the primary long-distance signal produced by water-stressed roots. Plant Signal. Behav. 2010, 5, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Vahisalu, T.; Kollist, H.; Wang, Y.-F.; Nishimura, N.; Chan, W.-Y.; Valerio, G.; Lamminmäki, A.; Brosché, M.; Moldau, H.; Desikan, R. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Lv, Y.; Wan, X.-R.; Li, L.-M.; Hu, B.; Li, L. Cloning and expression analysis of cDNAs encoding ABA 8′-hydroxylase in peanut plants in response to osmotic stress. PLoS ONE 2014, 9, e97025. [Google Scholar] [CrossRef]
- Stival Sena, J.; Giguère, I.; Rigault, P.; Bousquet, J.; Mackay, J. Expansion of the dehydrin gene family in the Pinaceae is associated with considerable structural diversity and drought-responsive expression. Tree Physiol. 2018, 38, 442–456. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Bullock, D.A., Jr.; Alonso, J.M.; Stepanova, A.N. To fight or to grow: The balancing role of ethylene in plant abiotic stress responses. Plants 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef] [Green Version]
- Gastauer, M.; Nascimento, W.R., Jr.; Caldeira, C.F.; Ramos, S.J.; Souza-Filho, P.W.M.; Féret, J.-B. Spectral diversity allows remote detection of the rehabilitation status in an Amazonian iron mining complex. Int. J. Appl. Earth Obs. Geoinf. 2022, 106, 102653. [Google Scholar] [CrossRef]
- Ramos, S.J.; Gastauer, M.; Martins, G.C.; Guedes, R.S.; Caldeira, C.F.; Souza-Filho, P.W.; Siqueira, J.O. Changes in soil properties during iron mining and in rehabilitating minelands in the Eastern Amazon. Environ. Monit. Assess. 2022, 194, 1–17. [Google Scholar] [CrossRef]
- Daghino, S.; Martino, E.; Perotto, S. Model systems to unravel the molecular mechanisms of heavy metal tolerance in the ericoid mycorrhizal symbiosis. Mycorrhiza 2016, 26, 263–274. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, M.; Sun, J.; Mao, X.; Wang, J.; Liu, H.; Zheng, H.; Li, X.; Zhao, H.; Zou, D. Heavy metal stress-associated proteins in rice and Arabidopsis: Genome-wide identification, phylogenetics, duplication, and expression profiles analysis. Front. Genet. 2020, 11, 477. [Google Scholar] [CrossRef]
- Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.V.; Bodanese Zanettini, M.H.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (HIPP): Characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Rono, J.K.; Sun, D.; Yang, Z.M. Metallochaperones: A critical regulator of metal homeostasis and beyond. Gene 2022, 822, 146352. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Liu, J.; Niu, Y.; Chen, Y.; Hao, Y.; Zhao, J.; Sun, L.; Wang, H.; Xiao, J. Characterization of the heavy-metal-associated Isoprenylated plant protein (HIPP) gene family from Triticeae species. Int. J. Mol. Sci. 2020, 21, 6191. [Google Scholar] [CrossRef]
- Singh, A.; Jha, S.K.; Bagri, J.; Pandey, G.K. ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis. PLoS ONE 2015, 10, e0125168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, R.J.; Ricachenevsky, F.K.; Fett, J.P. Differential regulation of the two rice ferritin genes (OsFER1 and OsFER2). Plant Sci. 2009, 177, 563–569. [Google Scholar] [CrossRef]
- Shevyakova, N.; Eshinimaeva, B.T.; Kuznetsov, V.V. Expression of ferritin gene in Mesembryanthemum crystallinum plants under different supply with iron and different intensity of oxidative stress. Russ. J. Plant Physiol. 2011, 58, 768–775. [Google Scholar] [CrossRef]
- Kasprzewska, A. Plant chitinases-regulation and function. Cell. Mol. Biol. Lett. 2003, 8, 809–824. [Google Scholar]
- Souleyre, E.J.; Bowen, J.K.; Matich, A.J.; Tomes, S.; Chen, X.; Hunt, M.B.; Wang, M.Y.; Ileperuma, N.R.; Richards, K.; Rowan, D.D. Genetic control of α-farnesene production in apple fruit and its role in fungal pathogenesis. Plant J. 2019, 100, 1148–1162. [Google Scholar] [CrossRef]
- Sattayachiti, W.; Wanchana, S.; Arikit, S.; Nubankoh, P.; Patarapuwadol, S.; Vanavichit, A.; Darwell, C.T.; Toojinda, T. Genome-wide association analysis identifies resistance loci for bacterial leaf streak resistance in rice (Oryza sativa L.). Plants 2020, 9, 1673. [Google Scholar] [CrossRef]
- Berka, M.; Kopecká, R.; Berková, V.; Brzobohatý, B.; Černý, M. Regulation of Heat Shock Proteins 70 and Their Role in Plant Immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef]
- Gong, B.-Q.; Wang, F.-Z.; Li, J.-F. Hide-and-seek: Chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef]
- Gough, C.; Cottret, L.; Lefebvre, B.; Bono, J.-J. Evolutionary history of plant LysM receptor proteins related to root endosymbiosis. Front. Plant Sci. 2018, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Kozaki, T.; Kouzai, Y.; Ozawa, K.; Ishii, K.; Asamizu, E.; Okabe, Y.; Umehara, Y.; Miyamoto, A.; Kobae, Y. The bifunctional plant receptor, OsCERK1, regulates both chitin-triggered immunity and arbuscular mycorrhizal symbiosis in rice. Plant Cell Physiol. 2014, 55, 1864–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari-Khorasgani, O.; Pessarakli, M. Breeding for Improved Plant–Symbiont Thermotolerance and Symbiotic Performance by Regulating Heat Shock Proteins, RNA Binding Proteins, and Chaperones. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 603–633. [Google Scholar]
- Takeda, N.; Sato, S.; Asamizu, E.; Tabata, S.; Parniske, M. Apoplastic plant subtilases support arbuscular mycorrhiza development in Lotus japonicus. Plant J. 2009, 58, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Grez, B.; Muñoz-Rojas, M.; Kariman, K.; Storer, P.; O’Donnell, A.G.; Kumaresan, D.; Whiteley, A.S. Reconditioning degraded mine site soils with exogenous soil microbes: Plant fitness and soil microbiome outcomes. Front. Microbiol. 2019, 10, 1617. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.C.; Kishi, L.T.; Lopes, E.M.; Omori, W.P.; Souza, J.A.M.d.; Alves, L.M.C.; Lemos, E.G.d.M. Bacterial communities in mining soils and surrounding areas under regeneration process in a former ore mine. Braz. J. Microbiol. 2018, 49, 489–502. [Google Scholar] [CrossRef]
- Al-Garni, S.M.S. Increased heavy metal tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. Afr. J. Biotechnol. 2006, 5, 133–142. [Google Scholar]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
| RM | Canga | |
|---|---|---|
| Organic carbon (g kg−1) | 8.2 ± 2.9 | 44.9 ± 5.7 |
| Organic matter (g kg−1) | 14.3 ± 5.0 | 77.4 ± 9.8 |
| pH (H2O) | 6.0 ± 0.17 | 4.65 ± 0.60 |
| Available P (mg dm−3) | 15.65 ± 3.55 | 1.0 ± 0.96 |
| Fe (mg dm−3) | 11.25 ± 4.19 | 372.5 ± 92 |
| Clay (g kg−1) | 488.7 ± 37.5 | 287.5 ± 103 |
| Sand (g kg−1) | 355 ± 64.5 | 625 ± 177 |
| Silt (g kg−1) | 162 ± 32.2 | 87.5 ± 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, S.V.d.; Herrera, H.; Costa, P.H.d.O.; Trindade, F.C.; da Costa, I.R.C.; Caldeira, C.F.; Gastauer, M.; Ramos, S.J.; Oliveira, G.; Valadares, R.B.d.S. Molecular Mechanisms Underlying Mimosa acutistipula Success in Amazonian Rehabilitating Minelands. Int. J. Environ. Res. Public Health 2022, 19, 14441. https://doi.org/10.3390/ijerph192114441
Nascimento SVd, Herrera H, Costa PHdO, Trindade FC, da Costa IRC, Caldeira CF, Gastauer M, Ramos SJ, Oliveira G, Valadares RBdS. Molecular Mechanisms Underlying Mimosa acutistipula Success in Amazonian Rehabilitating Minelands. International Journal of Environmental Research and Public Health. 2022; 19(21):14441. https://doi.org/10.3390/ijerph192114441
Chicago/Turabian StyleNascimento, Sidney Vasconcelos do, Héctor Herrera, Paulo Henrique de Oliveira Costa, Felipe Costa Trindade, Isa Rebecca Chagas da Costa, Cecílio Frois Caldeira, Markus Gastauer, Silvio Junio Ramos, Guilherme Oliveira, and Rafael Borges da Silva Valadares. 2022. "Molecular Mechanisms Underlying Mimosa acutistipula Success in Amazonian Rehabilitating Minelands" International Journal of Environmental Research and Public Health 19, no. 21: 14441. https://doi.org/10.3390/ijerph192114441








